The interdental brush allows for optimal plaque removal in the interproximal space, where the toothbrush cannot access and dental floss cannot work optimally.


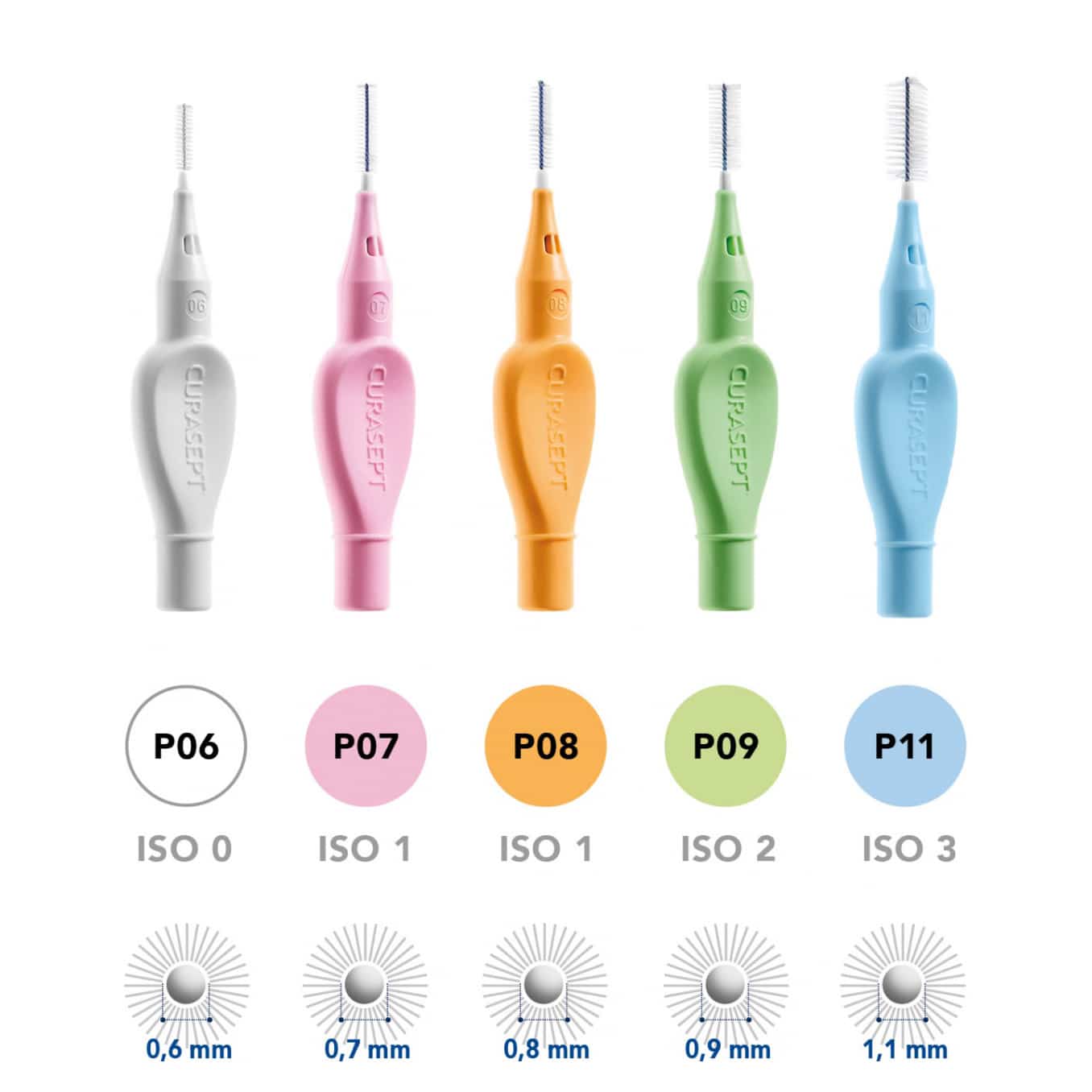
The spaces between teeth are not all the same, which is why there are different diameters.
The diameter of the brush (and therefore its adaptation to an ISO category) is given by the diameter of the metal core plus the bristles folded over it.

The size of an interdental brush is considered correct when the bristles completely fill the interdental space and the instrument enters the space without force.
Curasept Proxi range is made of
16 sizes available in the versions
- Straight
- Angular
The features of the two versions are the same, the Angle version may be more convenient for use in rear spaces.
To choose the right size it is useful and important to consult with your dentist or hygienist for advice.
In the meantime, you can experiment with the special Mix pack containing 5 interdental brushes of different sizes.