MANUAL OR SONIC?
The manual toothbrush is definitely the most widely used tool for oral hygiene.
However, in order to achieve the best cleaning action, the manual toothbrush must be used properly and must be chosen based on the shape of the head and the bristles, which must be suitable for any problem in your mouth.
The improper use, a wrong technique, too little time dedicated to brushing, too much pressure or hardness of the bristles, that can be the cause of
poor oral hygiene and cause damage to teeth and gums.
Sonic toothbrush can be considered as
a technological evolution of electric toothbrushes.
Its operation is based on high frequency
and on the range of movement of the bristles, which are
capable of generating a fluid-dynamic action.
The fluids present in the oral cavity at the time of use
spread evenly throughout the mouth
thanks to the rapid vibration of the bristles,
reaching even difficult place such as the interdental and molar areas.
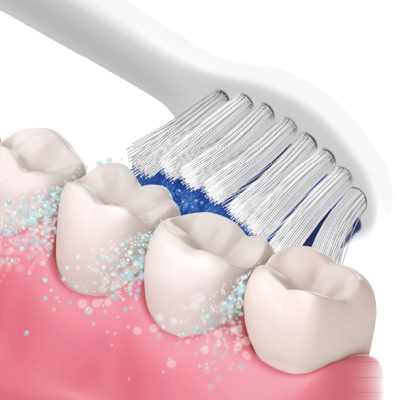
WHY CHOOSE A SONIC TOOTHBRUSH?
WHY CHOOSE A SONIC TOOTHBRUSH?
Those who choose the sonic toothbrush decide to revolutionize their dental hygiene, choosing an unparalleled technology and gentleness and previous.
The sonic toothbrush, thanks to the unique benefits of this technology, promises to take dental cleaning to unprecedented levels.
The sonic toothbrush is a safe tool and is suitable for everyone. Although extremely powerful against plaque, due to its gentleness it is also recommended for particular issues:
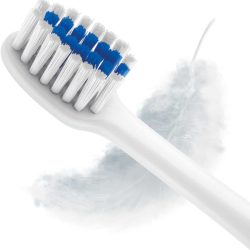
The sonic toothbrush does not require mechanical action in direct contact with enamel and gums and consequently helps to preserve their health. To use it and get a good result, just bring the toothbrush head close to your teeth.
The sonic vibrations enable a light massaging action that helps reactivate blood circulation in the gums.
The sonic toothbrush can reach those spaces between teeth where other toothbrushes cannot reach.
The 2-minute timer, divided into four 30-second fractions, allows you to use the toothbrush for the exact amount of time necessary for proper hygiene of the dental arches.
The different vibration intensities allow each user to choose the one best suited to his or her problem or sensitivity.